Full Length Research Paper - (2021) Volume 9, Issue 3
Studies on Abrus precatorius: An ethnoveterinary vine
Sandhya Deepika D1, Shyam Ch1*, Laxmi Sowmya K2 and Karimulla Sk12Department of Microbiology, Visakhapatnam, India
Abstract
Traditional folk treatment from wild plants has always guided researchers to search for novel medications to develop healthy life for humans and animals. Like most other plants Abrus precatorius contain various secondary metabolites with great potentials. The aim of this paper is to evaluate the phytochemicals, antioxidants and antimicrobial activity against veterinary pathogens by using quantitative and qualitative analysis of Hexane, Chloroform and Methanol with the help of standard techniques. The findings from quantification and phytochemical screening showed the presence of flavonoids, glycosides, cardiac glycosides, phenols, tannins, saponins, Proteins, Steroids and quinones. The in vitro antioxidant and antimicrobial activities of the specie, Abrus precatorius clearly demonstrated that whole plant have prominent antioxidant and antimicrobial properties.
Keywords
Abrus precatorius, Phytochemicals activity, Antioxidants activity, Antimicrobial activity.
Introduction
India is one of the World’s 12 regions having the largest biodiversity. It has 45000 plant species of which 15000-20000 m plants possess proven medicinal value (Krishna Kumar, 1996). Studies of plant taxonomies have to be updated. Biodiversity can be assessed and utilized only after determining the present state of the local and regional flora. A network of protected areas should be established to prevent the loss of the rich natural wealth. This would also help to preserve a range of ecotypes and a gene pool of medicinal plants. For many wild species of medicinal plants, no suitable cultural practices are known. Therefore, the domestication of species should be studied, especially of those that are under pressure. Biotechnology can help in the propagation of endangered species though it does not invent but uses already existing genes.
Ethnoveterinary practices cover people’s knowledge, skills, methods, practices and belief about the care of their animals (Mc Corkle, 1986). “Ethnoveterinary medicine” is the knowledge developed by local livestock holders and contrasts the allopathic veterinary medicine taught in Veterinary Colleges/Universities. Both are dynamic and changing. Ethno Veterinary Medicine is developed by farmers in field and barns rather than in scientific laboratories. It is less systematic, less formalized and usually transferred by word of mouth rather than writing. Ethno veterinary medicine is in the danger of extinction because of advancement of the modern veterinary medicine.
Limitations of Ethno Veterinary Practices:
1. Some remedies are inconvenient to prepare and use.
2. Availability of plants is seasonal.
3. Some practices are harmful.
4. Dosages are uncertain and remedies not standard (based on the empirical basis).
5. The diagnosis may be inadequate (as it is based on symptoms) rather than underlying cause of the disease.
6. Ethno medicines are not fast acting and potent and less suitable to treat epidemic and endemic infectious diseases e.g. suitable for digestive disorders.
Materials and Methods
Agricultural country like India Animal husbandry act as branch of agriculture concerned with the care and management of livestock. Animal husbandry deals with the feeding, breeding, housing and health care of livestock for getting maximum benefits. Livestock refers to farm animals (domesticated animals) such as cow, sheep, etc. kept by humans for a useful commercial purpose.
When we use the word “Animal” in animal husbandry, we mean only those domesticated animals which are reared mostly for economic or for recreation purposes, such as cattle, buffalo, sheep, goat, camel, pig, horse, etc. It also includes poultry farming and fisheries. The following organism’s causes major economic damages in the field of veterinary hence these five different strains of microorganisms were used in the screening listed in Table 1.
| Organism | NCIM Accession No |
|---|---|
| Brucella abortus | 5282 |
| Clostridium septicum | 2132 |
| Aspergillus fumigatus | 1272 |
| Candida albicans | 3102 |
| Salmonella enterica | 1164 (MTCC No) |
Aspergillosis
Aspergillosis is caused by several Aspergillus spp, especially A fumigatus and A terreus. A niger, A nidulans, A viridinutans, A flavus, and A felis are being recognized more commonly with increasing use of molecular techniques for identification. Aspergillosis can cause acute or chronic illness. Aspergillus infection is found worldwide and in almost all domestic animals and birds as well as in many wild species. It is primarily a respiratory infection that may become generalized; however, tissue predilection varies among species.
The most common forms are pulmonary infections in poultry and other birds; mycotic abortion in cattle; guttural pouch mycosis in horses; infections of the nasal and paranasal tissues, intervertebral sites, and kidneys of dogs; and sinonasal, sino-orbital, and pulmonary infection in domestic cats. In ruminants, aspergillosis may be asymptomatic, appear in a bronchopulmonary form, cause mastitis, or cause placentitis and abortion.
Mycotic pneumonia may be rapidly fatal. Signs include pyrexia; rapid, shallow, stertorous respiration; nasal discharge; and a moist cough. The lungs are firm, heavy, and mottled and do not collapse. In subacute to chronic mycotic pneumonia, the lungs contain multiple discrete granulomas, and the disease grossly resembles tuberculosis. Birds that are acutely infected will die quickly from respiratory distress. These birds may exhibit lethargy, dehydration, loss of appetite, diarrhoea, and may be gasping for breath before they die. Some birds may be found dead without observation of clinical signs. Acute infections are more common in younger birds, while older birds are more prone to chronic, slowly progressive disease.
Chronically infected birds may exhibit loss of body condition, lethargy, difficulty flying and breathing, vomiting, and diarrhoea. They may also show signs of neurological disease.
Brucellosis
Brucellosis is an infectious disease that occurs from contact with animals carrying Brucella abortus. It is one of the most common contagious and communicable zoonotic diseases with high rates of morbidity and lifetime sterility. Brucellosis is a widespread reproductive disease, commonly causing abortion, death of young ones, stillbirth, retained placenta or birth of weak calves, delayed calving, male infertility, and marked reduction in milk yield (Garofolo G et al., 2016; Celebi G et al., 2007).
It infects almost all domestic species except cats, which are naturally resistant to Brucella infection (Arif S et al., 2017). In bulls, the disease is characterized by fever, vesiculitis, orchitis, and epididymitis. In severe cases, it can also be the reason for testicular abscesses, metritis or orchitis that can lead to lifetime infertility. In animals, brucellosis symptoms can be varied from severe acute to sub-acute or chronic, depending upon the organ of infection and the type of animal (Currò V et al., 2012).
When a pregnant animal is infected by Brucella, a visible swelling of the mammary gland to the navel region and bleeding from the vagina is not uncommon, even if the cow does not abort. The enlarged udder size (appearance of the 9th month of a pregnant cow) could be used as an indication for the high stage of the disease, where animals shed bacteria in urine, milk, and vaginal discharges.
There has been a momentous increase over the recent years in intra/interspecific infection rates, due to poor management and limited resources, especially in developing countries. Abortion in the last trimester is a predominant sign, followed by reduced milk yield and high temperature in cattle.
Clostridia
Clostridia are the oldest known disease causing agent (pathogen) affecting livestock and other species. They are not contagious but are highly infectious and are ubiquitous worldwide. There are a large number of syndromes associated with clostridial disease in cattle and sheep, each with distinct risk factors, clinical signs and control. Braxy caused by C. septicum, which is normally found in gut and soil, this organism can proliferate particularly in animals fed autumn forage crops or roots that have been frosted. Disease is suspected to be due to frost damage to the lining of the abomasum (fourth stomach) which allows the organism to grow and produce toxin.
Clinical signs include sudden death or severe depression if found alive. Malignant Oedema is also caused by C. septicum (or occasionally C. chauvoei) which is found in gut and soil, this disease results from contamination of tissues due to deep penetrating wounds becoming infected with this bacterium. The organism proliferates in poorly perfused damaged tissue and elicits toxins which cause mortality-the animal is often just found dead. On post mortem examination tissues may be oedematous, with emphysema, malodour and swelling obvious. The main source of clostridia is spores in soil and regional differences in husbandry practices can affect incidence of disease.
The organisms are highly infectious but not contagious. After replication in the target organ, disease is often characterised by sudden death. The factors that influence disease occurrence depend on the species of clostridia involved. They may be ingested with feed and water and consequently healthy robust animals are often the first victims. The organisms are often present in organs or tissues of healthy animals and become pathogenic only after primary factors cause changes in habitat.
Examples of such primary factors include accidental injury, husbandry procedures, fighting, liver fluke infestation, overeating (too much starch) etc. It is advisable to look under each disease entity as epidemiology can differ for each.
Candidiasis
Candidiasis is a localized mucocutaneous disease caused by species of the yeast-like fungus Candida, most commonly C albicans. It is distributed worldwide in a variety of animals. C albicans is a normal inhabitant of the nasopharynx, Gastro Intestinal tract, and external genitalia of many species of animals and is opportunistic in causing disease. Factors associated with candidal infections are disruption of mucosal integrity; indwelling, intravenous, or urinary catheters; administration of antibiotics; and immunosuppressive drugs or diseases.
The organism most frequently infects birds, in which it involves the oral mucosa, esophagus, and crop. Superficial infections limited to the mucous membranes of the intestinal tract have been described in pigs and foals. Systemic candidiasis has also been described in cattle, calves, sheep, and foals secondary to prolonged antibiotic or corticosteroid therapy. In cats, candidiasis is rare but has been associated with oral and upper respiratory disease, pyothorax, ocular lesions, intestinal disease, and urocystitis. Infections are rare in dogs and horses. However, Candida spp have been considered a cause of arthritis in horses and mastitis and abortion in cattle. Fungemia and Candida peritonitis have been noted in dogs with perforating intestinal lesions after surgery, and mucosal and cutaneous candidiasis has been noted in immunosuppressed dogs and in dogs with diabetes mellitus.
Salmonella enterica
Salmonella enterica subspecies enterica can be separated into more than 2400 antigenically different serovars and the pathogenicity of most of these serovars is unspecified. The greater number of incidents of salmonellosis in humans and domestic animals originated from relatively few serovars and these can be separated into three groups on the basis of host prevalence. Host-specific serovars are the first group. These typically result in systemic disease in a small number of phylogenetically connected species. Host-restricted strains are the second group.
These are mainly connected with one or two closely related host species but may also unusually result with disease in other hosts. Animals become infected with Salmonella through their environment, by eating contaminated food, or from their mothers before they are even born or hatched. Salmonella is naturally in the intestines of many different animals. Animals with Salmonella shed the bacteria in their stool which can easily contaminate their body parts (fur, feathers, or scales) and anything in areas where these animals live and roam (terrarium or aquarium, chicken coop, pen or fencing, countertops, sinks, etc.). It is important to know that many animals can carry Salmonella and still appear healthy and clean.
Medicinal plants chosen for study
Abrus precatorius: Abrus precatorius belongs to the family Fabaceae commonly termed as Indian liquorice. It is a deciduous climbing plant producing numerous stems from the base that can be up to 6 metres, occasionally 9 metres long. These stems scramble over the ground, twining into other nearby plants for support. The brightly coloured seeds are popularly used as beads to make necklaces and rosaries. Although poisonous, they also have a wide range of medicinal applications. The seeds contain a number of medically active ingredients, including the extremely toxic substance abrin, indole alkaloids and anthocyanins. Hey are extremely toxic but are used medicinally as an abortifacient, contraceptive, emetic and irritant. The seeds are also antiperiodic, bitter, aphrodisiac, diaphoretic, emetic, expectorant and purgative. They have played an important role in the treatment of conjunctivitis in various parts of the world. The leaves and roots contain glycyrrhizin and minute amounts of the toxin abrin. They have soothing properties and are expectorant, anti- inflammatory and ant allergic.
Preparation of plant extract
Plants are complex matrices, producing a range of secondary metabolites with different functional groups and polarities. Although water is used as an extractant in many traditional protocols. Organic solvents of varying polarities are generally selected in modern methods of extraction to exploit the various solubilities of plant constituents. Soxhlet extraction is widely used for both initial and bulk extraction. Its main advantage is that the material is extracted continuously, i.e., the solvent saturated in solubilized metabolites empties into the flask, fresh re-condensed solvent then re-extracts the material in the thimble. This method is less time and solvent consuming than maceration or percolation.
100 g of ground weighed material of fine coarse powder was successively extracted by different solvents of hexane, chloroform and methanol, in a specific sequence based on increasing polarity. The soxhlet hot extraction procedure for each of the above solvents was run for about 6 hours, until a colourless solvent was seen in the siphon tube, which indicated complete extraction. The solvents were removed under reduced pressure and controlled temperature by rotary evaporator. The extracts were dried and stored in a clean glass bottle and kept at 4°C-6°C for further antimicrobial screenings at 4°C till further investigation.
Maceration
4 Kg of dried leaves were macerated using 8.6 L methanol in 1 L conical flasks at room temperature for 24 hours. Then the extracts obtained were filtered using Whatman filter paper to obtain methanol extract. The residue left was again subjected to second successive extraction with methanol, following previously mentioned procedure to get the second methanol extract. Then both extracts were studied for their TLC profiles and owing to their similar TLC pattern they were mixed. Thus obtained methanolic extract was concentrated in rotary flash evaporator and dried in a vacuum oven so as to obtain thick, viscous mass. Then the yield value was calculated and the concentrated methanolic extract was subjected to the successive extraction using hexane, chloroform and methanol to obtain hexane, chloroform and methanol soluble.
Phytochemical tests
The phytochemical test of these extracts was performed using the method adopted by Harborne and Sofowora.
1. Test for carbohydrates (Molisch’s test): To 2 ml of plant extract, 1 ml of Molisch’s reagent and a few drops of concentrated sulfuric acid were added. Presence of purple or reddish color indicates the presence of carbohydrates.
2. Test for tannins (Ferric chloride test): To 1 ml of plant extract, 2 ml of 5% ferric chloride was added. Formation of dark blue or greenish black indicates the presence of tannins.
3. Test for saponins (Frothe’s test): To 2 ml of plant extract, 2 ml of distilled water was added and shaken in a graduated cylinder for 15 minutes lengthwise. Formation of a 1 cm layer of foam indicates the presence of saponins.
4. Test for flavonoids (Shinoda test): To 2 ml of plant extract, 1 ml of 2N sodium hydroxide was added. Presence of yellow color indicates the presence of flavonoids.
5. Test for alkaloids (Mayer’s test): To 2 ml of plant extract, 2 ml of concentrated hydrochloric acid was added. Then a few drops of Mayer’s reagent were added. The presence of green color or white precipitate indicates the presence of alkaloids.
6. Test for quinones: To 1 ml of extract, 1 ml of concentrated sulphuric acid was added. Formation of red color indicates presence of Quinones.
7. Test for glycosides (Molisch’s test): To 2 ml of plant extract, 3 ml of chloroforms and 10% ammonia solution was added. Formation of pink colour indicates presence of glycosides.
8. Test for cardiac glycosides (Keller–Kiliani test): To 0.5 ml of extract, 2 ml of glacial acetic acid and a few drops of 5% ferric chloride were added. This was under layered with 1 ml of concentrated sulfuric acid. The formation of brown ring at the interface indicates presence of cardiac glycosides.
9. Test for terpenoids (Salkowski test): To 0.5 ml of extract, 2 ml of chloroform was added and concentrated sulfuric acid is added carefully. Formation of red brown color at the interface indicates presence of terpenoids.
10. Test for phenols (Ferric chloride test): To 1 ml of the extract, 2 ml of distilled water followed by a few drops of 10% ferric chloride was added. Formation of blue or green color indicates presence of phenols.
11. Test for proteins: To the 0.5 ml of extract, 0.5 ml of extract, 2 ml of 40% sodium hydroxide and 1 ml 1% CuSO4 were added formation of pink colour indicates presence of proteins.
DPPH radical scavenging assay
Free radical scavenging is one of the mechanisms involved in antioxidant action, a good antioxidant (AH) able to scavenge the DPPH (1,1 Di phenyl 2-picryl hydrazyl) radical and retain its own stability due to its reduction ability as shown in the equation below.
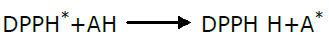
Procedure
Plant extracts were tested for the scavenging effect on DPPH radical method, 2 ml of extract solution of different solvents (Hexane, Chloroform and Methanol) were taken in different concentration (5, 50, 100 and 400 μg) to which 2 ml of 0.4 mM/L DPPH methanolic solution was added. Solution containing 2 ml of methanol and 2 ml of the DPPH solution was used as negative control and synthetic antioxidant ascorbic acid was used as positive control. Different concentrations were kept in the dark at room temperature for 30 min. The scavenging activity of the DPPH was determined by measuring the absorbance at 517 nm until the reaction reached the steady state, using a spectrophotometer. All the determination was performed five times.
The DPPH radical scavenging activity was calculated using the following equation.
%inhibition=(1-A1/A0) × 100
A1 and A0 are the absorbance of the tested sample and control respectively.
Ferric reducing power assay
Ferric reducing/antioxidant power (FRAP) was determined following the method as described earlier by Zhao H et al., Briefly, 100 μl of each concentration of the extracts (100-600 μg/ml) was mixed with 2.5 ml of 200 mM phosphate buffer (pH 6.6) and 2.5 ml of 1% potassium ferricyanide and incubated at 50°C for 20 min. After this, 2.5 ml of 10% trichloroacetic acid was added and the tubes were centrifuged at 10,000 rpm for 10 min. Five milliliters of the upper layer of the solution was mixed with 5.0 ml of distilled water and 1 ml of 0.1% ferric chloride and the absorbance of the reaction mixtures was measured at 700 nm. The final results were expressed as mg ascorbic acid equivalent/g of dry weight.
%Inhibition=[(Control absorbance-Sample absorbance)/ Control absorbance] × 100
Ferric reducing antioxidant power
The antioxidant capacity of the medicinal plants was estimated spectrophotometrically following the procedure of Benzie and Strain. The method is based on the reduction of Fe3+ TPTZ complex (colorless complex) to Fe2+-tripyridyltriazine (blue colored complex) formed by the action of electron donating antioxidants at low pH. This reaction is monitored by measuring the change in absorbance at 593 nm.
The Ferric reducing antioxidant power (FRAP) reagent was prepared by mixing 300 mM acetate buffer, 10 ml TPTZ in 40 mM HCl and 20 mM FeCl3.6H2O in the proportion of 10:1:1 at 37°C. Freshly prepared working FRAP reagent was pipetted using 1-5 ml variable micropipette (3.995 ml) and mixed with 5 μl of the appropriately diluted plant sample and mixed thoroughly. An intense blue color complex was formed when ferric tripyridyl triazine (Fe3+TPTZ) complex was reduced to ferrous (Fe2+) form and the absorbance at 593 nm was recorded against a reagent blank (3.995 ml FRAP reagent+5 μl distilled water) after 30 min incubation at 37°C.
All the determinations were performed in triplicates. The calibration curve was prepared by plotting the absorbance at 593 nm versus different concentrations of FeSO4. The concentrations of FeSO4 were in turn plotted against concentration of standard antioxidant trolox. The FRAP values were obtained by comparing the absorbance change in the test mixture with those obtained from increasing concentrations of Fe3+ and expressed as mg of Trolox equivalent per gram of sample.
in vitro antimicrobial assays
The development of simple in vitro pre-screens could offer initial idea of the biological activity of plant extracts and its compounds. Two types of media, solid (agar) and liquid (broth) media are generally required for culturing of microbes and bioassay studies. This solid matrix, by preventing any significant mixing of colonies of the culture, is best for presenting microbes on the surface of a medium, as needed for isolation of pure colonies, the liquid medium, on the other hand is the more mobile form of the culture and is most useful for creating concentrated inocula of the microbe. The antimicrobial activity was performed by using two methods-Agar disc diffusion for essential oil extracts and Agar ditch or well diffusion method for solvent extracts. Of these two methods the agar well diffusion assay was used to screen for the anti-microbial activity of extracts of different plant species. Which is the most widely used type for identifying the antimicrobial activity, which exploit diffusion of antimicrobial compounds through agar media to demonstrate the inhibition of bacteria and fungi.
Antibacterial activity
Composition of nutrient agar medium:
Peptone : 5 g
Meat extract : 10 g
Sodium chloride : 5 g
Agar agar : 15 g
Distilled water : 1000 mL
pH adjusted to : 7.2 to 7.4
Agar cup plate method/Agar well diffusion method
In agar well diffusion method peptone (0.5 grams), meat extract (1.0 grams), sodium chloride (0.5 grams) and agar (1.5 grams) were dissolved in small quantity of distilled water with the aid of heat on water bath and the volume was made up to 100 mL with purified water. The pH of the nutrient broth was adjusted to 7.2 using 5 M sodium hydroxide, and then sterilized in an autoclave maintained at 121 degree centigrade (15 lbs.) for 20 minutes.
After sterilization, the medium was inoculated with 3 μL aliquots of culture containing approximately 105 CFU/mL of each organism of 24 hours slant culture in aseptic condition and transferred into sterile petri dishes and allowed to set at room temperature for about 10 minutes and then kept in a refrigerator for 30 minutes. After setting a number 3 cup borer (6 mm) diameter was properly sterilized by flaming and used to make four to five uniform cups/wells in each petri dish. A drop of molten nutrient agar was used to seal the base of each cup. The cups/wells were filled with 50 μL of the different extracts of 100 mg/mL, 250 mg/mL, and 500 mg/mL so final drug concentration will be 5 mg/well, 15 mg/well, and 25 mg/well respectively and allow diffusing of plant extract into the medium for about 45 minutes.
Standard drugs ciprofloxacin (10 μg/mL), control (0.1% DMSO) were transferred to the cups of each agar plate by means of sterile pipettes under a laminar flow unit. The plates thus prepared were left for 2 hours in refrigerator for diffusion and then kept in an incubator at 37 degree centigrade. After 24 hours, the agar plates were examined for inhibition zones, and the zones were measured in millimetres. The zones of inhibition were measured with antibiotic zone scale in mm and the experiment was carried out in triplicates.
Determination of Minimum Inhibitory Concentration (MIC)
The MIC values of extracts were determined based on a micro broth dilution method in 96 multi-well microtiter plates with slight modifications. For susceptibility testing, 1 gm of plant material powder were initially dissolved in 1000 μL of dimethyl sulfoxide (DMSO) to reach final concentration of 1 g/mL; 100 μL of this suspension were added to the first test well of each microtiter line, as sterility control and then 50 μL of scalar dilutions were transferred from the second to the tenth well. The 11th well was considered as growth control, since no extracts solutions were added. 50 μL of broth was distributed from the second to the twelveth well. Then, 50 μL of the bacterial suspensions were added to each well. Plates were incubated for 24 h at 37°C. Ciprofloxacin (10 μg/mL), was prepared and used as standard drug for positive control. As an indicator of bacterial growth, 40 μL piodonitrotetrazolium (INT) violet dissolved in water was added to the wells and incubated at 37°C for 30 min. The lowest concentration of extract showing no growth was taken as its minimal inhibitory concentration (MIC) and confirmed by plating 5 μL samples from clear wells on agar medium. The tetrazolium salt acts as an electron acceptor and is reduced to a red-colored formazan product by biologically active organisms. Where bacterial growth was inhibited, the solution in the well remained clear after incubation with INT.
Antifungal activity
Composition of Potato dextrose agar medium:
Potatoes (peeled) : 200.0 g
Dextrose : 20.0 g
Agar-Agar : 15.0 g
Distilled water : 1000 mL
Procedure
Peeled potatoes (20 grams) were cut into small pieces and boiled with 100 mL of water for 30 minutes. The pieces are crushed during boiling and the pulp was removed after cooling by filtration through muslin cloth. Dextrose (2 grams) and agar (1.5 grams) were added and the volume is made up to 100 mL. And the medium was then sterilized in an autoclave at 121 degree centigrade (15 lbs.) for 30 min. After sterilization the medium was inoculated by using 4 days culture of the test organisms by adjusted the turbidity was equivalent to 0.5 McFarland units (equivalent to 1.5 × 105 or 106 CFU/mL) in aseptic condition and transferred to sterile petri dishes and allowed to set at room temperature for about 10 minutes. Four to five cups of 6 mm diameter bore in medium at equal distance were made in each agar plate by using sterile borer.
Methanol extracts in different concentrations (100 mg/mL, 250 mg/mL, and 500 mg/mL) to get the final drug concentration 5 mg/well, 15 mg/well, and 25 mg/well respectively, controls (DMSO) and standard (Fluconazole, 10 μg/mL), was transferred to the cups of each agar plate. The plates thus prepared were left for 2 hours for diffusion and then incubated at room temperature 28°C. After 36 hours, the agar plates were examined for inhibition zones, and the zones were measured in millimetres. The zones of inhibition were measured with antibiotic zone scale in mm and the experiment was carried out in triplicates.
Minimum Fungicidal Concentration (MFC)
The Minimum Inhibitory Concentration (MIC) of different extracts was determined by microdilution method using serially diluted (2 folds) plant extract according to the National Committee for Clinical Laboratory Standards (NCCLS) (National Committee for Clinical Laboratory Standards). Equal volume of each extract and broth were mixed in test tubes. Specifically, 0.1 mL of standardized inoculums (1-2 × 105 CFU/mL) was added in each tube. The tube was incubated aerobically at 37°C for 24 to 48 hours. Two control tubes were maintained for each test batch, these included antifungal control (tube containing extract and growth media without inoculums) and organism control (tube containing the growth medium, saline and the inoculums). The lowest concentration (highest dilution) of the extract that produced no visible fungal growth (no turbidity) when compared with the control tube was regarded as MIC. However, the MFC was determined by sub culturing the test dilution on to PDA medium and incubated further for 72 hrs. The highest dilution that yielded no fungal colony on the solid medium was taken as MFC.
Results and Discussion
Yield value determination
The plant extractives were analyzed to estimate the percentage yield of individual extracts and found that, the yield was abundant in methanol rather than chloroform and hexane. Due to the high polarity of methanol most of the chemical constituents of extracts would be dissolved in it and thus percentage yield was increased tremendously than other solvents. Due to higher yield in methanol, methanolic extracts were selected for further studies. Yield was highest in Soxhlet Extraction method when compared with the Maceration. Yield was more in methanol followed by hexane and chloroform (Table 2).
| S.NO | Plant name | Yield value (%) | |||||
|---|---|---|---|---|---|---|---|
| Hexane | Chloroform | Methanol | |||||
| Soxhlet Extraction | Maceration | Soxhlet Extraction | Maceration | Soxhlet Extraction | Maceration | ||
| 1 | Abrus precatorius | 33.81 | 28 | 18.01 | 11 | 43.62 | 39.46 |
Loss on drying
The loss of weight was calculated as the content in mg per g of air-dried material. The percent loss on drying was then calculated for each plant powder. Percentage of loss on drying for Abrus precatorius was 2.29%.
Ash values
In the evaluation of crude extracts, total ash value is particularly important in the evaluation of purity of drugs, i.e. the presence or absence of foreign inorganic matter such as metallic salts or silica. The amount and composition of ash remaining after combustion of plant material varies considerably according to the part of the plant, age, treatment etc. The constituents of ash also vary with time and from organ to organ. Ash usually represents the inorganic part of the plant. The total ash method is designed to measure the total amount of material remaining after ignition. The total ash content of Abrus precatorius was 3.12%.
Preliminary phytochemical analysis
Abrus precatorius was a rich source of secondary metabolites such as flavonoids, glycosides, cardiac glycosides, phenols, tannins, saponins, Proteins, Steroids and quinones (Table 3).
| S. NO. | Phytochemical test | Abrus precatorius |
|---|---|---|
| 1 | Alkaloids | - |
| 2 | Flavanoids | + |
| 3 | Glycosides | + |
| 4 | Cardiac glycosides | ++ |
| 5 | Tanins | +/- |
| 6 | Phenols | + |
| 7 | Steroids | + |
| 8 | Quniones | ++ |
| 9 | Proteins | + |
| 10 | Carbohydrates | - |
| 11 | Saponins | ++ |
Anti-oxidant studies
Flavonoids are class of secondary plant metabolites with significant antioxidant and chelating properties. Antioxidant activity of flavonoids depends on the structure and substitution pattern of hydroxyl groups. Total Flavonoids content in the Plant extracts expressed interms of Rutin equivalent (mg of RUE/g of extract). The total flavonoid content was more in the Chloroform (56.66 ± 0.05 RUE/g) followed by Methanol (56.17 ± 0.09 RUE/g) and Hexane (52.05 ± 0.08 RUE/g). The total phenolic contents in the examined extracts ranged from 23.86 ± 0.02 to 65.66 ± 0.03 mg GAE/gm.
The highest concentration of phenols was measured in Methanol (65.66 ± 0.03 GAE/gm) followed by Chloroform (47.39 ± 0.07 GAE/gm) and Hexane (23.86 ± 0.02 GAE/gm).
Ferric Reducing Antioxidant Power (FRAP) assay
FRAP values were higher in methanol extracted samples compared to hexane and chloroform extraction. This showed that methanol extraction was more efficient in extracting antioxidants in plant materials compared to water. The FRAP values of Abrus precatorius are represented in Table 4 below.
| S. NO. | Plant name | Hexane | Chloroform | Methanol |
|---|---|---|---|---|
| 1 | Abrus precatorius | 37.21 ± 0.02 | 42.07 ± 0.07 | 77.25 ± 0.03 |
Free radical scavenging activity by DPPH assay
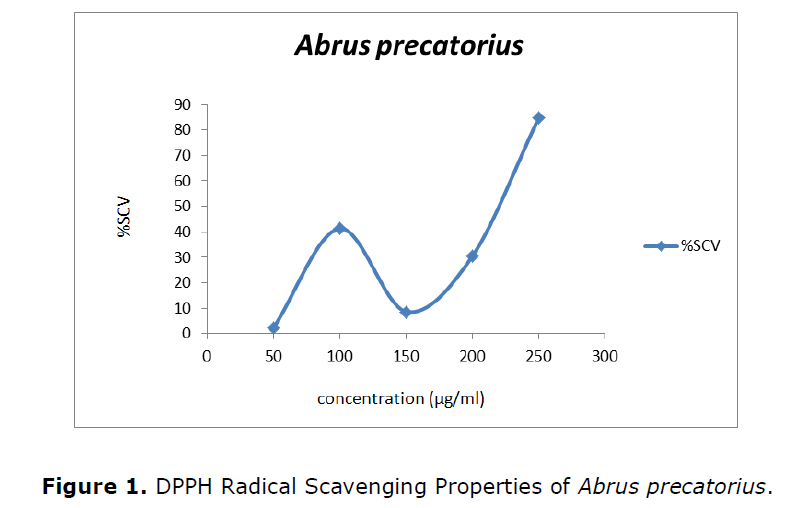
The antioxidant activity of different plant extracts was determined using a methanol solution of DPPH reagent. The antioxidant activity was expressed in terms of percentage of inhibition (%). The effect of antioxidants on DPPH is thought to be due to their hydrogen donating ability. The DPPH activity was represented in Figure 1 and the IC50 value of the Abrus precatorius was 210.08.
Antimicrobial activity
The medicinal plant experimental data only methanol extracts exhibited moderate to high inhibition zones (activity) against all the microflora screened, Hexane and Chloroform extracts showed only mild to no activity, hence only methanol extracts reports were analyzed.
Abrus precatorius at 100 mg/ml showed maximum activity on Brucella abortus and Candida albicans (15 mm) followed by Salmonella enterica (13 mm), Clostridium septicum (10 mm) and least activity was observed in Aspergillus fumigatus (9 mm) (Table 5).
| S. No. | Organism | Zone of inhibition (mm) | |||||
|---|---|---|---|---|---|---|---|
| 200 mg/ml | 150 mg/ml | 100 mg/ml | MIC | Standard | Control | ||
| 1 | Brucella abortus | 15 | 13 | 10 | 25 | 37 | 6 |
| 2 | Clostridium septicum | 10 | 8 | - | 50 | 37 | 6 |
| 3 | Salmonella enterica | 13 | 10 | 9 | 25 | 36 | 6 |
| 4 | Aspergillus fumigatus | 9 | 7 | 11 | 20 | 36 | 6 |
| 5 | Candida albicans | 15 | 15 | 9 | 25 | 34 | 6 |
Discussion
Ethnoveterinary medicine is the name given to the way in which most livestock keepers in Cameroon and other countries treat animal health problems. Ethnoveterinary practices are important because they are easily available, inexpensive and effective, especially in rural areas where veterinary services are absent or irregular and expensive.
At this level, indigenous animal health systems are used for emergency purposes. Until 1989, ethnoveterinary practices were mostly carried out at individual level, with little coordination. In 1989 the Cameroon ethnoveterinary Council was founded. This council has about 300 members, all practicing ethnoveterinary. Bringing ethnoveterinary together allows members to share ideas and work together, for example creating ethnoveterinary gardens, doing research and gathering knowledge.
India has a wide variety of flora and fauna, where flora contains about 6,000 species of phanerogams. In India the local populations of different regions have century's old knowledge and traditional practices of most of the plants occurring in those regions. This local knowledge of plants has been transferred from generation to generation through verbal communication and personal experience.
In the present study Abrus precatorius was used for the analysis of phytochemicals, antioxidant activity and antimicrobial activity against five selected veterinary pathogens i.e., Brucella abortus, Clostridium septicum, Salmonella enterica, Aspergillus fumigatus and Candida albicans.
Phytochemical screening was performed for analysing secondary metabolites, which were believed to be responsible for useful pharmacological effects with therapeutic application. The preliminary phytochemical screening for secondary metabolites was carried out on petroleum ether and methanol (70%) seed extracts of A. precatorius using standard procedures. The phytochemicals tested for were alkaloids, flavonoids, carbohydrates, tannins, steroids, proteins, triterpenes, glycosides, saponins, and fat and oils. Presence and absence of phytochemical constituents were noted by Shazia Tabasum et al., Amit Saraf et al. noted that the plant is a good source of secondary metabolites. It also indicates that ethanol and methanol are the best solvents among various solvents used for extraction of secondary metabolites from the plant under study Marimuthu et al., recorded aqueous extract contains phytochemicals such as alkaloid, flavonoid, carbohydrate, protein, amino acid and steroid Hussain and Kumaresan evaluated for preliminary phytochemical screening standard procedure. Preliminary phytochemical screening showed the presence of various classes of secondary metabolites such as alkaloids, flavonoids, phenols, saponins, glycosides, tannins, carbohydrate and terpenoids. Tania Tabassum et al., recorded Phytochemical analysis of methanolic extract of the plant showed the presence of alkaloids, tannins, gum and glycosides and the absence of flavonoid and saponins. Abrus precatorius possesses high antioxidant activity. Nassi et al., gathered experimental evidence that ethanolic extract of Iraqi plant Abrus precatorius contained diverse phytochemicals especially the alkaloids, flavonoids, steroids, and terpenoids and tannins, and the absence of, anthraquinoin and cardiac glycosides.
Several studies have pronounced a positive correlation between phenolic content and antioxidant activity using similar assay system, but in present study could not establish correlation in similar manner.
It could be due to the existing of other reducing compounds have been established in the ethanolic extract of Abrus precatorius by phytochemical analysis, these compounds, nonphenolic in its nature, like terpenoids and alkaloids with antioxidant effects. The different compositions of extract which one of each have different antioxidant potency that make some of them having synergistic effect or they react quickly with DPPH while the other compounds have a slower reaction mechanism and require to high concentrations to have significant effect.
Bobbarala V et al., 2009 worked on the methanolic crude extracts showed maximum antibacterial activity on Klebsilla pneumonia, followed by Staphylococcus aureus, Streptococcus mitis and Micrococus luteus, respectively. The studied plants were most active against all the bacteria tested. The significant antibacterial activity of the active plant extracts was comparable to the standard antibiotic Streptomycin (10 μg/disc) (Roy et al., 2012). Aqueous extracts of raw seeds of Abrus precatorius showed antibacterial effect on both Gram positive (Bacillus subtilis MTCC 121 and Staphylococcus aureus MTCC96), as well as Gram negative bacteria (Pseudomonas aeruginosa MTCC 741 and Salmonella typhimurium MTCC98) by Adelowotan et al., 2008; Sreeramulu et al., 2009 focused on antibacterial activity by using the following strains of gram positive bacteria (Staphylococcus aureus and Bacillus subtilis) and gram negative (Escherichia coli and Salmonella typhi). In addition to this antifungal activity is also carried out by using the following strains of Candida albicans, Cryptococcus neoformans. Though methanol soluble and insoluble fractions exhibited moderate activity against gram positive bacteria, it had no effect against gram negative bacteria. Like antibacterial activity, antifungal activity indicated that the crude extract of white form significantly affected fungal growth than red form although fractions of both forms showed moderate activity.
Conclusion
The above organisms are not only a threat to livestock but also a global public health issue. Unfortunately, we lack not only a proper treatment but also a reliable diagnosis. Adequate and timely diagnosis of the infection is necessary to control and treat the disease in the best way.
Different serological and molecular methods are used for the screening of the disease. However, each test has some drawbacks in one way or another. So here we suggest that due to the zoonotic importance of the infection, it is necessary to handle the disease in a proper way and a combination of particular tests should be used to screen for brucellosis in both humans and animals. The different cited studies regarding zoonotic infections in humans and cattle revealed that the combination of both the molecular and serological methods must be practiced for accurate diagnosis. If the infected animals are in chronic infected condition, they should be culled to prevent the disease spreading. The formal education and necessary training of farmers, especially those living in rural areas, would also help to get control over the disease.
Methanolic extracts of Abrus precatorius showed good activity against five selected veterinary pathogens i.e., Brucella abortus, Clostridium septicum, Salmonella enterica, Aspergillus fumigatus and Candida albicans. Further work is needed to locate the active principle from the various extracts and their phyto pharmaceutical studies. Research into the effects of local medicinal plants is expected to boost the use of these plants in the therapy against disease caused by the test bacterial species and other microorganisms. There are immense opportunities for the development of the drugs on the basis of the ethno botanical leads. These drugs can be used for the effective animal health care at affordable cost to the peasantry of the country. Besides, the development of the Ethno veterinary practices would also provide income generation to the marginal farmers of the country. However, there is an urgent need of the amalgamation of the modern veterinary medicine. Modern science and the Ethno veterinary practices as to derive synergy in the Animal health care.
References
- Krishna Kumar PR (1996). Indian Medicine Industry under the emerging patent regimes. Anc. Sci. Life. 15: 161.
- Mc Corkle CM (1986). An introduction to ethno Veterinary Research and development. J. Ethnobiol. 6(1):129-149.
- Garofolo G, Fasanella A, Di Giannatale E, Platone I, Sacchini L, Persiani T, Boskani T, Rizzardi K, Wahab T (2016). Cases of human brucellosis in Sweden linked to Middle East and Africa. BMC Res. Notes. 9:277.
- Celebi G, Külah C, Kiliç S, ÜstündaÄ? G (2007). Asymptomatic Brucella bacteraemia and isolation of Brucella melitensis biovar 3 from human breast milk. Scand. J. Infect. Dis. 39:205-208.
- Arif S, Thomson CP, Hernandez-Jover M, McGill MD, Warriach MH, Heller J (2017) Knowledge, attitudes and practices (KAP) relating to brucellosis in smallholder dairy farmers in two provinces in Pakistan. PLoS ONE. 12:e0173365.
- Currò V, Marineo S, Vicari D, Galuppo L, Galluzzo P, Nifosì D, Pugliese M, Migliazzo A, Torina A, Caracappa S (2012). The isolation of Brucella spp. from sheep and goat farms in Sicily. Small Rumin. Res. 106:S2-S5.
- Bobbarala V, Vadlapudi V(2009). Abrus precatorius l. seed extracts antimicrobial properties against clinically important bacteria.Int. J. PharmTech Res.1(4):1115-1118.
- Roy S, Acharya R, Mandal NC, Barman S, Ghosh R, Roy R (2012). A comparative antibacterial evaluation of raw and processed GuñjÄ (Abrus precatorius Linn.) seeds Anc. Sci. Life. 32(1):20-23.
- Adelowotan O, Aibinu I, Adenipekun E, Odugbemi T (2008). The in-vitro antimicrobial activity of Abrus precatorius (L) fabaceae extract on some clinical pathogens. Niger. Postgrad. Med. J. 15(1):32-37.
- Sreeramulu J, Raveendra reddy J, Padmanabha reddy Y, Geethavani M (2009) Antimicrobial Activity of Seeds of Abrus precatorius Linn. Asian J. Chem. 21(2): 1630-1632.