Mini Review - (2022) Volume 10, Issue 1
Potential of algae in holistic wellness of pets
Neera Chugh, Supriya Iyer, Manish R. Shukla and Santanu Dasgupta*Received: 18-Jan-2022, Manuscript No. AASTGB-22-42308; Editor assigned: 21-Jan-2022, Pre QC No. AASTGB-22-42308 (PQ); Reviewed: 04-Feb-2022, QC No. AASTGB-22-42308; Revised: 11-Feb-2022, Manuscript No. AASTGB-22-42308 (R); Published: 21-Feb-2022, DOI: 10.51268/2736-1810- 22.10.056
Abstract
Algae have drawn great attention as a sustainable alternative protein ingredient in feed industry. While high protein content is considered to be the main reason behind its popularity, presence of valuable bioactive compounds like PUFA, carotenoids, polysaccharides, minerals, vitamins etc. offers several possible health benefits to the animals. This review discusses key algae based feed ingredients and its beneficial effect on overall health of animals fed with algae based diets, with a focus on pet animals.
Keywords
Algae, Feed ingredient, Bioactive compounds, Pet health.
Introduction
Rapid shift towards pet parenting worldwide has a paramount impact on the pet food industry (Schleicher et al., 2019). Alltech global feed survey reports a 4% rise in the pet feed production globally (Alltech, 2020). According to Mintel reports, 91% pet parents in the US consider pets as their family members (Future Market Insights, 2021). This trend of humanization of pets has heightened the concerns about pet health and nutrition. Besides pet owners demanding protein rich diets, there has been an increasing awareness about high quality functional foods with proactive ingredients, that possibly can provide benefits like boosting immunity, bone health, gut health and also contributes to the overall wellness of pets (Dust et al., 2005; Korucu, 2018). Traditionally pet food industry has wide range of protein sources including, poultry by product, bone meal, meat, fish, and some plant ingredients such as corn, gluten, and soymeal (Thompson, 2008). In response to enhanced consumer awareness regarding issues of pet health, nutrition and sustainability, search for alternative novel feed ingredients has increased in the past few years.
Proteins are the most expensive and limiting ingredients in feed formulation. It mainly provides nitrogen and essential amino acids (EAA) in dogs and cats (NRC, 2006). Identification of additional sources of nitrogen and amino acids, including taurine which is an essential dietary requirement in cats (AAFCO, 2013), will help meet the dietary requirements of canines and feline thereby reducing the competition for food resources. Use of plant (Hill and PAS, 2004), insects (Bosch et al., 2014) and algae (McCusker et al., 2014) as a novel ingredient or additive in dogs and cat diets have been extensively studied lately. While the plant based ingredients competes directly with human food, on the other hand insect and algae derived ingredients are not dependent on arable land and thus provide sustainability driven advantage in meeting the needs of the animal feed industry.
Algae are known to be an excellent source of proteins, carotenoids, Omega- fatty acids and contain several bioactive molecules (Camacho et al., 2019; Rajvanshi et al., 2019). Spirulina and Chlorella are reported as nutritional products, used as supplements, treats or as complete foods for dogs and cats (Beynen, 2019). However, incorporation of new ingredients in animal diets requires careful assessment as it can have varied impact on the overall growth, nutrition, metabolism, micro biota of the gut and immune response towards diseases. This review mainly focuses on potential of algae in providing holistic wellness when used as supplement food in pet diet.
Algae As Protein Ingredients in Pets
Protein plays a vital role in the overall development of pets, allowing muscle growth, maintaining fur, skin, and nails and making critical components of the immune system. Growing consumer awareness on nutrition, health, and sustainability (McCusker et al., 2014) more and more pet owners are considering natural or organic ingredients in the pet diets.
Amongst plant based protein sources soybean meal is the supplemental protein source most widely used in animal feeds. Soybean meal is considered as an excellent source of protein (Fan et al., 1995). However, soybean based ingredients may possess undesirable qualities such as, anti-nutritional factors or imbalance in amino acid composition (Gatlin et al., 2007). Study on soybean meal reports that concentrations greater than 150 g/kg in canine foods have adverse effect on the digestibility (Yamka et al., 2003).
Microalgae have gained focus as an alternative protein ingredient in feed industry (Becker, 2013; Enzing et al., 2014; Camacho et al., 2019). High protein content and presence of essential amino acids profile comparable to existing protein ingredients makes microalgae a lucrative alternative to animal based protein (Barka and Blecker, 2016; Madeira et al., 2017). In general crude protein in microalgae is variable and usually ranges between 40% to 60% on dry weight basis depending on species and environmental conditions (Becker, 2007). On the basis of protein contents some of the green and blue green algae are reported as supplement for pet food (Beynen, 2019).The Association of American Food Control Officials (AAFCO) and European Pet Food Industry Federation (FEDIAF) had laid minimum 18%-25% protein requirement for dog and cat food during growth and reproduction/adult maintenance stage (AAFCO, 2013; FEDIAF, 2019). Apart from the protein requirement that can easily be met by microalgae, compared to soymeal microalgae has lower trypsin inhibition activity (anti- nutritional factor), indicating the use of microalgae in feed without any major concerns of anti- nutritional factors (Subhash et al., 2020).
Role of Algae in Gut Health
Gastrointestinal tract of mammals harbors a biodiverse group of microorganisms that play a vital role on pathological and physiological state of animal. Alterations in gut- micro biota are associated with inflammation, disease, obesity, metabolic disorder etc. (Mondo et al., 2019).The role of microalgae in promoting gut health is well reported in humans and animals (Fields et al., 2020; Becker, 2013). Lactic acid bacteria are found in canine gut micro biota (Handl et al., 2011) and lactic acid as probiotics have a safe and effective history in dogs (Benyacoub et al., 2003; Vahjen and Männer, 2003). Spirulina extracts are known to increase the growth of lactic acid bacteria (Parada et al., 1998). Additionally, marine algae are also known to produce soluble polysaccharides which escape conventional digestion and are fermented by the gut micro biota thereby providing substrates for micro biota growth (Michel and Macfarlane, 1996). A recent study reported supplementation with 0.2% spray-dried Spirulina in diets of dogs resulted in improving intestinal micro biota stability (Satyaraj et al., 2021). Although inclusion of microalgae and its effect on dynamics of gut micro biota is widely explored for aquaculture (Shah et al., 2018) and livestock animals (Madeira et al., 2017). Research on the benefits of inclusion of microalgae on gut micro biota of canine and feline is still at its infancy and needs further study to understand the role of microalgae on intestinal health.
Algae With Immunomodulatory Activity
Recently, algal compounds are reported to stimulate the immune system in human and mice models (Heo et al., 2012). Table 1 represents algae derived compounds with immune modulatory activity (Guzman et al., 2003; Kwak et al., 2012; Barsanti and Gualtieri, 2019; Hirahashi et al., 2002; Cerezuela et al., 2012; Pugh et al., 2001; Bhardwaj et al., 2021; Yeganeh and Adel, 2019; Risjani et al., 2021).
| Algae species | Components | Target animals/Humans |
|---|---|---|
| Chlorella stigmatophora | Crude polysaccharide | Rats |
| Chlorella vulgaris | 97% pure algae powder | Human trials |
| Euglena gracilis | Beta-Glucans | Human trials |
| Spirulina sp. | Hot water extract | Human trials |
| Tetraselmis chuii | Lyophilized microalgae Powder | Gilthead seabream (Sparus aurata L.) |
| Nannochloropsis gaditana | Lyophilized microalgae Powder | Gilthead seabream (Sparus aurata L.) |
| Aphanizomenon flosaquae | Algal polysaccharide | Human trials |
| Chlorella pyrenoidosa | Algal polysaccharide | Human trials |
| Spirulina platensis | Algal polysaccharide | Human trials |
| Phaeodactylum tricornutum | Crude Polysaccharide | Rats |
| Turbinaria ornata | Sulfated polysaccharide | Rats |
| Sargassum ilicifolium | Dry Powder | Juvenile great sturgeon Huso huso L |
| Porphyridium cruentum | Polysaccharide | Zebrafish and White Shrimp Litopenaeus vannamei. |
Micro-Algal Immune Modulators in Pets
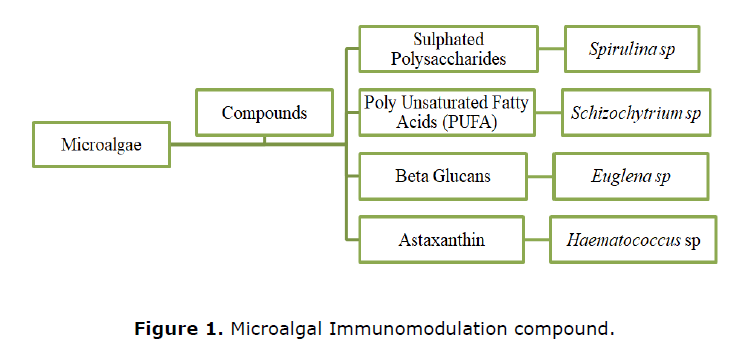
Microalgae play important role in animal nutrition from aquaculture to farm animals (Sathasivam et al., 2019). Microalgae have been reported for improving the immune system, lipid metabolism, gut function, and providing stress resistance in livestock and aquaculture feeds (Shields and Lupatsch, 2012). In recent years, research on novel and natural compounds as immune modulators from algae is gaining popularity in mammals (Ricco and Lauritano, 2020). Figure 1 represents the compounds derived from microalgae that are reported to have immune modulatory activity. This section gives a brief overview of algae derived compounds reported as immune modulators in pets.
Sulfate polysaccharides (sPS)
Microalgal polysaccharides have been shown to have antiviral, antioxidative, anti-inflammatory, and immunomodulatory activity (Raposo et al., 2013). A study reported anticancer property of Spirulina on hematopoietic system of mouse and dogs which were exposed with cyclophosphamide and 60Co-Ɣ irradiation. The polysaccharide extracted from Spirulina platensis had chemo protective and radio protective capability and was explained to be the potential agent for restoration of hematopoiesis in mice and dogs (Zhang et al., 2001).
Spirulina is reported to enhance the macrophage functions and IL-1 production without changing IgG-antibody production in mice. A study, in cats, dogs as well as in humans, reports phycocyanin and water soluble polysaccharides extracted from Spirulina platensis was reported to enhance the macrophage functions (Raja and Hemaiswarya, 2010).
Polyunsaturated Fatty Acids (PUFAs)
Microalgae are considered as important source of polyunsaturated fatty acids and they are known to synthesize both omega 6 and omega 3 fatty acids. Considering the continuous reduction of available fishery and seafood resources, microalgae have been evaluated as a sustainable and eco-friendly alternative for PUFA (Khozin-Goldberg et al., 2016).Omega 3 FAs are considered to be essential during all life stages of dogs (AAFCO, 2013). Study reported on brain function of aged beagle dogs suggest that consumption of a diet fortified with DHA-rich algae (0.4% dried whole-cell algal biomass) helped maintained a healthy brain function (Hadley et al., 2017). In Beagle dogs, incorporation of algal oil containing EPA and DHA (AOCED) at dietary levels up to 3.0 % wt., equivalent to 30 g/kg diet in extruded dry food was found to be safe during gestation, lactation, and growth periods (Dahms et al., 2019) Another study suggests that addition of 0.4% microalgae as a source of DHA in diets of Beagle dogs showed, enhanced palatability, higher amount of monocytes and phagocytic granulocytes as compare to control and demonstrated oxidative stability which was superior to anchovy oil (Souza et al., 2019).
Beta glucans
β-Glucans are linear or branched glucose polymers found in the cell walls of fungi, yeasts, bacteria, algae, mushrooms and as callose in higher plants (Stone, 2004; Odabasi et al., 2006; Thompson et al., 2010). Beta-1, 3-glucan in dogs helps maintain a healthy immune system by supporting production of cytokine, white blood cells and antibodies (Kataoka et al., 2002; Stuyven et al., 2010). Microalgae such as Euglena gracilis can accumulate up to 80% (w/w) of its dry weight of reserve polysaccharide paramylon, known as β-1, 3-glucan (Sun et al., 2018; Barsanti and Gualtieri, 2019).
Study on immunomodulatory effects of β-1, 3/1, 6-glucan on leukocyte functions and vaccination responses in 6 weeks old puppies suggested that oral administration of 4 mg/kg soluble glucans daily once resulted in increased phagocytic activity of leukocytes and enhanced antibody levels of against rabies as well as canine parvovirus type 2 in comparison to control (Vojtek et al., 2017).
Carotenoids
Astaxanthin is carotenoid synthesize by Haematococcus pluvialis and reported to be a powerful antioxidant (Panis and Carreon, 2016). Various studies demonstrate the advantage of using astaxanthin as a supplement in pet diets (Beynen, 2020). A study on uptake of astaxanthin in domestic dogs and cats reported that biokinetic uptake of astaxanthin in dogs and cats are similar to that in humans (Park et al., 2010). In geriatric female dogs, dietary intake of 20 mg astaxanthin daily for 16 weeks reduced oxidative and inflammatory damage and consequently enhanced mitochondrial function suggesting the role of astaxanthin in modulating age-associated mitochondrial dysfunction (Park et al., 2013). In another study, dietary supplementation of astaxanthin (0.3 mg/kg body weight/day) for 6 weeks in healthy dogs and 8 weeks in diets of obese beagle dogs improved liver function by enhancing lipid metabolism (Murai et al., 2019).
Benefits of Algae on Skin, Oral and Vision of Pets
In pets, inflammation is a primary biological defense response of the body against any external microorganisms. In vivo studies on the use of Chlorella extract supplemented in diets of beagle with chronic dermatitis showed anti-inflammatory effects with disappearance of inflammation in the auricle and other affected areas and overall improvement in dermatitis after 4 weeks (Maeda et al., 2008).
Periodontal disease is a very common disease in dogs (Wallis et al., 2019) responsible for deposition of calculus on tooth enamel and plaque formation, which leads to tooth loss, gingivitis, and periodontitis (Harvey, 2005). A study reported positive clinical effects of Ascophyllum nodosum supplementation in form of edible treats containing 25% w/w alga for 90 days, showed improvement in dental health indices, prevented plaque and calculus formation after a prophylactic dental procedure. Additionally, Ascophyllum nodosum was reported to reduce levels of volatile sulfur compounds thereby providing long-term good oral health in dogs (Gawor et al., 2018).
Keratoconjunctivitis sicca (cKCS) in canines is an inflammatory eye condition that affects cornea and conjunctiva and is related to deficiency in tear aqueous fraction (Barnett and Joseph, 1987). Combination of classical drug therapy with a nutraceutical diet containing Ascophyllum nodosum, and Astaxanthin from Haematococcus pluvialis, with other natural plant based components having potential anti-inflammatory and immune-modulating activities, showed reduction in clinical symptoms of keratoconjunctivitis sicca (Destefanis et al., 2016).
Conclusion
Increased consumer awareness on the nutrition and overall health of pets has resulted in increased use of probiotics and synthetic immune modulators. Algae beyond its basic role to provide an alternative protein source, offers additional benefits as immunoboosters, antiviral and antibacterial agent that can possibly help in the overall wellbeing of pets. Thereby reducing the use of other feed additives and making it an economically viable ingredient in the pet food formulation. Although use of algae derived compounds in pet food is limited to only few species and is at a very nascent stage. Further research on effectively utilizing various bioactive compounds of algae is needed. Information on its safety, bioavailability and palatability should be investigated prior to incorporation into canine and feline diets as a partial replacement or as a supplement in pet food.
Acknowledgements
The authors would like to acknowledge Reliance Industries Limited (RIL) for support.
Conflict of Interest
The author(s) declare(s) that there is no conflict of interest regarding the publication of this article.
REFERENCES
Schleicher M, Cash SB, Freeman LM (2019). Determinants of pet food purchasing decisions. Can. Vet. J. 60(6): 644. [GoogleScholar] [Pubmed]
Alltech (2020). 2020 Global feed survey.
Future Market Insights (2021). High Protein Dog Food Market.
Dust JM, Grieshop CM, Parsons CM, Karr-Lilienthal LK, Schasteen CS, Quigley III JD, Merchen NR, Fahey Jr GC (2005). Chemical composition, protein quality, palatability, and digestibility of alternative protein sources for dogs. J. Anim. Sci. 83(10): 2414-2422. [Crossref] [GoogleScholar] [Pubmed]
Korucu A (2018). 5 trends that influence the pet food industry.
Thompson A (2008). Ingredients: where pet food starts. Top. Companion. Anim. Med. 23(3): 127-132. [Crossref] [GoogleScholar] [Pubmed]
National Research Council (2006).Nutrient requirements and dietary nutrient concentration. In: Nutrient Requirements of Dogs and Cats. Washington, DC: National Academy Press, pp. 354-370. [Crossref] [GoogleScholar] [Pubmed]
AAFCO. (2013). AAFCO Methods for Substantiating Nutritional Adequacy of Dog and Cat Foods.
Hill D, Pas D (2004). Alternative proteins in companion animal nutrition. In: Pet Food Association of Canada Fall Conference.[GoogleScholar]
Bosch G, Zhang S, Oonincx DG, Hendriks WH (2014). Protein quality of insects as potential ingredients for dog and cat foods. J. Nutr. Sci. 3. [Crossref] [GoogleScholar] [Pubmed]
McCusker S, Buff PR, Yu Z, Fascetti AJ (2014). Amino acid content of selected plant, algae and insect species: a search for alternative protein sources for use in pet foods. J. Nutr. Sci. 3: e39. [Crossref] [GoogleScholar] [Pubmed]
Camacho F, Macedo A, Malcata F (2019). Potential Industrial Applications and Commercialization of Microalgae in the Functional Food and Feed Industries: A Short Review. Mar. Drugs. 17(6): 312. [Crossref] [GoogleScholar] [Pubmed]
Rajvanshi M, Sagaram US, Subhash GV, Kumar GRK, Kumar C, Govindachary S, Dasgupta S (2019). Biomolecules from microalgae for commercial applications. In: Sustainable Downstream Processing of Microalgae for Industrial Application. CRC Press. pp. 3-38. [GoogleScholar]
Beynen AC (2019). Microalgae in petfood. Creat. Companion. 40(7). [GoogleScholar]
Fan MZ, Sauer WC, De Lange CF (1995). Amino acid digestibility in soyabean meal, extruded soyabean and full-fat canola for early-weaned pigs. Anim. Feed Sci. Tech. 52(3-4): 189-203. [Crossref]
Gatlin III DM, Barrows FT, Brown P, Dabrowski K, Gaylord TG, Hardy RW, Herman E, Hu G, Krogdahl Å, Nelson R, Overturf K (2007). Expanding the utilization of sustainable plant products in aquafeeds: a review. Aquac. Res. 38(6): 551-579. [Crossref][GoogleScholar]
Yamka RM, Jamikorn U, True AD, Harmon DL (2003). Evaluation of soyabean meal as a protein source in canine foods. Anim. Feed Sci. Technol. 109(1-4): 121-132. [Crossref][GoogleScholar]
Becker EW (2013). Microalgae for Human and Animal Nutrition. In: Richmond A, Qiang Hu, Handbook of Microalgal Culture. Wiley Online Library.
Enzing C, Ploeg M, Barbosa M, Sijtsma L (2014). Microalgae-based products for the food and feed sector: an outlook for Europe. JRC. Sci. policy. rep. 19-37. [GoogleScholar]
Barka A, Blecker C (2016). Microalgae as a potential source of single-cell proteins: A review. Base. 20:3. [Crossref] [GoogleScholar] [Pubmed]
Madeira MS, Cardoso C, Lopes PA, Coelho D, Afonso C, Bandarra NM, Prates JA (2017). Microalgae as feed ingredients for livestock production and meat quality: A review. Livest. Sci. 205: 111-121.
Becker EW (2007). Micro-algae as a source of protein. Biotechnol. Adv. 25(2): 207-210. [Crossref] [GoogleScholar] [Pubmed]
FEDIAF (2019). Nutritional Guidelines for Complete and Complementary Pet Food for Cats and Dogs. European Federation of the Pet Food Industry.
Subhash GV, Chugh N, Iyer S, Waghmare A, Musale AS, Nandru R, Dixit RB, Gaikwad MS, Menon D, Thorat R, Kumar GR (2020). Application of in vitro protein solubility for selection of microalgae biomass as protein ingredient in animal and aquafeed. J. Appl. Phycol. 32(6): 3955-3970. [Crossref][GoogleScholar]
Mondo E, Marliani G, Accorsi PA, Cocchi M, Di Leone A (2019). Role of gut microbiota in dog and cat’s health and diseases. Open Vet. J. 9(3): 253-258. [Crossref] [GoogleScholar] [Pubmed]
Fields FJ, Lejzerowicz F, Schroeder D, Ngoi SM, Tran M, McDonald D, Jiang L, Chang JT, Knight R, Mayfield S (2020). Effects of the microalgae Chlamydomonas on gastrointestinal health. J. Funct. Foods. 65: 103738. [Crossref][GoogleScholar]
Handl S, Dowd SE, Garcia-Mazcorro JF, Steiner JM, Suchodolski JS (2011). Massive parallel 16S rRNA gene pyrosequencing reveals highly diverse fecal bacterial and fungal communities in healthy dogs and cats. FEMS Microbiol. Ecol. 76(2): 301-310. [Crossref] [GoogleScholar] [Pubmed]
Benyacoub J, Czarnecki-Maulden GL, Cavadini C, Sauthier T, Anderson RE, Schiffrin EJ, von der Weid T (2003). Supplementation of food with Enterococcus faecium (SF68) stimulates immune functions in young dogs. J. Nutr. Sci. 133(4): 1158-1162. [Crossref] [GoogleScholar] [Pubmed]
Vahjen W, Männer K (2003). The effect of a probiotic Enterococcus faecium product in diets of healthy dogs on bacteriological counts of Salmonella spp., Campylobacter spp. and Clostridium spp. in faeces. Arch. Anim. Nutr. 57(3): 229-233. [Crossref] [GoogleScholar] [Pubmed]
Parada JL, de Caire GZ, de Mulé MC, de Cano MM (1998). Lactic acid bacteria growth promoters from Spirulina platensis. Int. J. Food Microbiol. 45(3): 225-228. [Crossref] [GoogleScholar] [Pubmed]
Michel C, Macfarlane GT (1996). Digestive fates of soluble polysaccharides from marine macroalgae: involvement of the colonic microflora and physiological consequences for the host. J. Appl. Microbiol. 80(4): 349-369. [Crossref] [GoogleScholar] [Pubmed]
Satyaraj E, Reynolds A, Engler R, Labuda J, Sun P (2021). Supplementation of diets with Spirulina influences immune and gut function in dogs. Front. Nutr. 8: 267. [Crossref] [GoogleScholar] [Pubmed]
Shah MR, Lutzu GA, Alam A, Sarker P, Chowdhury MK, Parsaeimehr A, Liang Y, Daroch M (2018). Microalgae in aquafeeds for a sustainable aquaculture industry. J. Appl. Phycol. 30(1): 197-213. [Crossref][GoogleScholar]
Heo SJ, Yoon WJ, Kim KN, Oh C, Choi YU, Yoon KT, Kang DH, Qian ZJ, Choi IW, Jung WK (2012). Anti-inflammatory effect of fucoxanthin derivatives isolated from Sargassum siliquastrum in lipopolysaccharide-stimulated RAW 264.7 macrophage. Food Chem Toxicol. 50(9): 3336-3342. [Crossref] [GoogleScholar] [Pubmed]
Guzman S, Gato A, Lamela M, Freire‐Garabal M, Calleja JM (2003). Anti‐inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother Res. 17(6): 665-670. [Crossref] [GoogleScholar] [Pubmed]
Kwak JH, Baek SH, Woo Y, Han JK, Kim BG, Kim OY, Lee JH (2012). Beneficial immunostimulatory effect of short-term Chlorella supplementation: enhancement of natural killer cell activity and early inflammatory response (randomized, double-blinded, placebo-controlled trial). Nutr. J. 11(1): 1-8. [Crossref] [GoogleScholar] [Pubmed]
Barsanti L, Gualtieri P (2019). Paramylon, a potent immunomodulator from wzsl mutant of Euglena gracilis. Molecules. 24(17): 3114. [Crossref] [GoogleScholar] [Pubmed]
Hirahashi T, Matsumoto M, Hazeki K, Saeki Y, Ui M, Seya T (2002). Activation of the human innate immune system by Spirulina: augmentation of interferon production and NK cytotoxicity by oral administration of hot water extract of Spirulina platensis. Int. Immunopharmacol. 2(4): 423-434. [Crossref] [GoogleScholar] [Pubmed]
Cerezuela R, Guardiola FA, Meseguer J, Esteban MA (2012). Enrichment of gilthead seabream (Sparus aurata L.) diet with microalgae: effects on l immune system. Fish Physiol. Biochem. 38(6): 1729-1739. [Crossref] [GoogleScholar] [Pubmed]
Pugh N, Ross SA, ElSohly HN, ElSohly MA, Pasco DS (2001). Isolation of three high molecular weight polysaccharide preparations with potent immunostimulatory activity from Spirulina platensis, Aphanizomenon flos-aquae and Chlorella pyrenoidosa. Planta Med. 67(8): 737-742. [Crossref] [GoogleScholar] [Pubmed]
Bhardwaj M, Mani S, Malarvizhi R, Sali VK, Vasanthi HR (2021). Immunomodulatory Activity of Brown Algae Turbinaria ornata Derived Sulfated Polysaccharide on LPS Induced Systemic Inflammation. Phytomedicine. 153615. [Crossref] [GoogleScholar] [Pubmed]
Yeganeh S, Adel M (2019). Effects of dietary algae (Sargassum ilicifolium) as immunomodulator and growth promoter of juvenile great sturgeon (Huso huso Linnaeus, 1758). J. Appl. Phycol. 31(3): 2093-2102. [Crossref][GoogleScholar]
Risjani Y, Mutmainnah N, Manurung P, Wulan SN (2021). Exopolysaccharide from Porphyridium cruentum (purpureum) is Not Toxic and Stimulates Immune Response against Vibriosis: The Assessment Using Zebrafish and White Shrimp Litopenaeus vannamei. Mar. Drugs. 19(3): 133. [Crossref] [GoogleScholar] [Pubmed]
Sathasivam R, Radhakrishnan R, Hashem A, Abd Allah EF (2019). Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 26(4): 709-722. [Crossref] [GoogleScholar] [Pubmed]
Shields R, Lupatsch I (2012). 5 Algae for aquaculture and animal feeds. In: De Gruyter, Microalgal Biotechnology: Integration and Economy. pp: 79-100. [Crossref]
Riccio G, Lauritano C (2020). Microalgae with immunomodulatory activities. Mar. Drugs. 18(1): 2. [Crossref] [GoogleScholar] [Pubmed]
Raposo MF, De Morais RM, Bernardo de Morais AM (2013). Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs. 11(1): 233-252. [Crossref] [GoogleScholar] [Pubmed]
Zhang HQ, Lin AP, Sun Y, Deng YM (2001). Chemo and radio-protective effects of polysaccharide of Spirulina platensis on hemopoietic system of mice and dogs. Acta Pharmacol. Sin. 22(12): 1121-1124.
Raja R, Hemaiswarya S (2010). Microalgae and immune potential. In: Dietary Components and Immune Function. Humana Press, Totowa, New Jersey, pp. 515-527. [Crossref][GoogleScholar]
Khozin-Goldberg I, Leu S, Boussiba S (2016). Microalgae as a source for VLC-PUFA production. In: Lipids in plant and algae development. 471-510. [Crossref] [GoogleScholar] [Pubmed]
Hadley KB, Bauer J, Milgram NW (2017). The oil-rich alga Schizochytrium sp. as a dietary source of docosahexaenoic acid improves shape discrimination learning associated with visual processing in a canine model of senescence. Prostaglandins Leukot. Essent. Fatty Acids. 118: 10-18. [Crossref] [GoogleScholar] [Pubmed]
Dahms I, Bailey-Hall E, Sylvester E, Parenteau A, Yu S, Karagiannis A, Roos F, Wilson J (2019). Safety of a novel feed ingredient, Algal Oil containing EPA and DHA, in a gestation-lactation-growth feeding study in Beagle dogs. PLoS One. 14(6): e0217794. [Crossref] [GoogleScholar] [Pubmed]
Souza CM, de Lima DC, Bastos TS, de Oliveira SG, Beirão BC, Félix AP (2019). Microalgae Schizochytrium sp. as a source of docosahexaenoic acid (DHA): Effects on diet digestibility, oxidation and palatability and on immunity and inflammatory indices in dogs. Anim. Sci. J. 90(12): 1567-1574. [Crossref] [GoogleScholar] [Pubmed]
Stone BA (2004). Cellulose and callose: evolutionary considerations. In: abstracts of papers of the american chemical society. Northwest Washington, District of Columbia, United States. Amer. Chemical Soc. 227: U289-U289.
Odabasi Z, Paetznick VL, Rodriguez JR, Chen E, McGinnis MR, Ostrosky-Zeichner L (2006). Differences in beta-glucan levels in culture supernatants of a variety of fungi. Med. Mycol. J. 44(3): 267-272. [Crossref] [GoogleScholar] [Pubmed]
Thompson IJ, Oyston PC, Williamson DE (2010). Potential of the beta-glucans to enhance innate resistance to biological agents. Expert Rev. Anti Infect. Ther. 8(3): 339-352. [Crossref] [GoogleScholar] [Pubmed]
Kataoka K, Muta T, Yamazaki S, Takeshige K (2002). Activation of macrophages by linear (1→ 3)-β-d-glucans: implications for the recognition of fungi by innate immunity. J. Biol. Chem. 277(39): 36825-36831. [Crossref] [GoogleScholar] [Pubmed]
Stuyven E, Verdonck F, Van Hoek I, Daminet S, Duchateau L, Remon JP, Goddeeris BM, Cox E (2010). Oral administration of beta-1,3/1,6-glucan to dogs temporally changes total and antigen-specific IgA and IgM. Clin. Vaccine. Immunol. 17(2): 281-285. [Crossref] [GoogleScholar] [Pubmed]
Sun A, Hasan MT, Hobba G, Nevalainen H, Te'o J (2018). Comparative assessment of the Euglena gracilis var. saccharophila variant strain as a producer of the β‐1, 3‐glucan paramylon under varying light conditions. J. Phycol. 54(4): 529-538. [Crossref] [GoogleScholar] [Pubmed]
Vojtek B, Mojžišová J, Smrčo P, Drážovská M (2017). Effects of orally administered β–1, 3/1, 6–glucan on vaccination responses and immunological parameters in dogs. Food Agric. Immunol. 28(6): 993-1002. [Crossref][GoogleScholar]
Panis G, Carreon JR (2016). Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res. 18: 175-190. [Crossref][GoogleScholar]
Beynen AC (2020). Krill in pet food. Creat. Companion. 40-41.
Park JS, Kim HW, Mathison BD, Hayek MG, Massimino S, Reinhart GA, Chew BP (2010). Astaxanthin uptake in domestic dogs and cats. Nutr. Metab. 7(1): 1-8. [Crossref] [GoogleScholar] [Pubmed]
Park JS, Mathison BD, Hayek MG, Zhang J, Reinhart GA, Chew BP (2013). Astaxanthin modulates age-associated mitochondrial dysfunction in healthy dogs. J. Anim. Sci. 91(1): 268-275. [Crossref] [GoogleScholar] [Pubmed]
Murai T, Kawasumi K, Tominaga K, Okada Y, Kobayashi M, Arai T (2019). Effects of astaxanthin supplementation in healthy and obese dogs. Vet. Med. 10: 29-35. [Crossref] [GoogleScholar] [Pubmed]
Maeda M, Kasuya Y, Yuasa K, Hiranaka T, Chubachi H, Saito M, Takegoshi H (2008). The anti-inflammatory activity of Chlorella in beagles with skin disorders. Proc Annual Meeting Japan Society for Bioscience, Biotechnology and Agrochemistry. [Crossref]
Wallis C, Pesci I, Colyer A, Milella L, Southerden P, Holcombe LJ, Desforges N (2019). A longitudinal assessment of periodontal disease in Yorkshire terriers. BMC Vet. Res. 15(1): 1-11. [Crossref] [GoogleScholar] [Pubmed]
Harvey CE (2005). Management of periodontal disease: understanding the options. Vet. Clin. Small Anim. 35(4): 819-836. [Crossref] [GoogleScholar] [Pubmed]
Gawor J, Jank M, Jodkowska K, Klim E, Svensson UK (2018). Effects of Edible Treats Containing Ascophyllum nodosum on the Oral Health of Dogs: A Double-Blind, Randomized, Placebo-Controlled Single-Center Study. Front. vet. sci. 5: 168. [Crossref] [GoogleScholar] [Pubmed]
Barnett KC, Joseph EC (1987). Keratoconjunctivitis sicca in the dog following 5-aminosalicylic acid administration. Hum. Toxicol. 6(5): 377-383. [Crossref] [GoogleScholar] [Pubmed]
Destefanis S, Giretto D, Muscolo MC, Di Cerbo A, Guidetti G, Canello S, Giovazzino A, Centenaro S, Terrazzano G (2016). Clinical evaluation of a nutraceutical diet as an adjuvant to pharmacological treatment in dogs affected by Keratoconjunctivitis sicca. BMC Vet. Res. 12(1): 1-12. [Crossref][GoogleScholar] [Pubmed]